UreaseThe method described here uses bromothymol blue indicator which changes from yellow to blue as ammonia is released during the reaction.
Urease, (EC3.5.1.5), from the jack bean (Canavalia ensiformis) was the first enzyme to be crystalised, for which discovery James Sumner was awarded a Nobel Prize for Chemistry in 1946. It breaks down urea into carbon dioxide and ammonia. A number of tests for urease utilise the fact that the ammonia released causes a rise in pH which can be detected with suitable indicators. The method described here gives good results with continuous colorimetry using bromothymol blue indicator which undergoes a change from yellow to blue as the pH rises from 6 to 7.6. The reaction can be followed by measuring the change in absorbance of red light with the colorimeter. Other indicators can be used. Phenol red works well and bicarbonate indicator can be used, but bromothymol blue is our preferred choice.
Absorbance vs Time 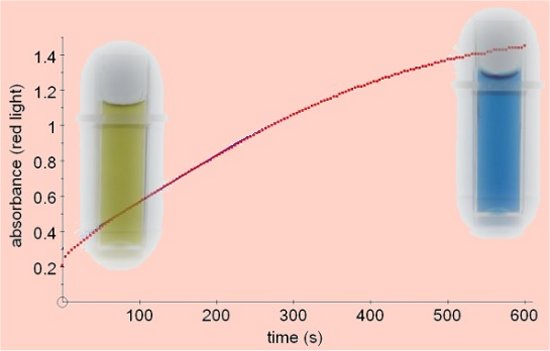
Reaction mixture
Jack bean urease is commercially available in powder or tablet form - the enzyme should be freshly prepared as it does not seem to keep well. The results shown (absorbance versus time) were obtained at 25°C using 3cm3 of 10mM urea containing 0.01% bromothymol blue and 0.2cm3 of an enzyme solution made by adding one urease tablet to 80cm3 of water.  Click to download a pdf file containing much of the information about this
topic.
Click to download a pdf file containing much of the information about this
topic.
|