Dopa oxidase
The experiments with dopa oxidase are easy, safe, inexpensive, reliable and colourful. In our opinion this is the ideal system for investigating and understanding many of the properties of enzymes.
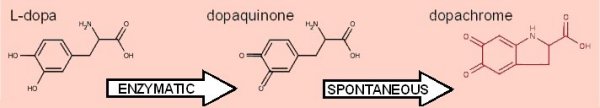
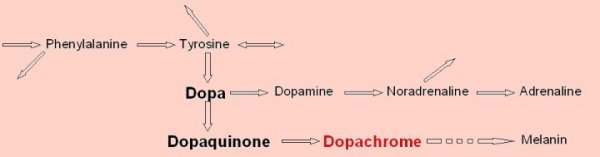
Although it is a considerable oversimplification of what is a rather complex system, (see below), the basic reaction studied is as shown here.
The conversion of L-dopa, (3,4-dihydroxy-L-phenylalanine), to dopachrome is one of the easiest, safest, most reliable and inexpensive enzyme reactions we have encountered. It is linked to other reactions and
illustrates nicely the concept of biochemical pathways. A number of interesting and significant compounds are closely associated with the reaction, for example,
-
the amino acid tyrosine is an alternate substrate for the reaction, though it is considerably slower and not particularly recommended. The synthesis of tyrosine from phenylalanine is the reaction that is blocked
in PKU, the inborn error of metabolism that all new-born babies are tested for. The condition can be treated through environmental modification, by
controlling the intake of dietary phenylalanine. Many foods are marked with phenylalanine content for this reason.
-
dopachrome, the end point of the assay described here, is the pigment responsible for the hair colour of red-heads.
-
dopachrome undergoes further conversion to yield melanin, the dark pigment in skin and hair. An inability to manufacture melanin causes albinism.
dopa is converted to dopamine, then to the neurotransmitter noradrenaline and to adrenaline. This function has led to dopa being used to treat
Parkinson’s disease
. It is sold as a component of dietary supplements with a startling variety of claims to improve, amongst others, everything from the immune system and energy levels to lean muscle
mass and sexual performance.
At first sight this appears to be an incredibly complicated, controversial and incompletely understood enzyme system, a complexity that is reflected in the multiplicity of names. Indeed it is unclear just how many
enzymes are involved in the phenolase complex.
EC 1.14.18.1
monophenol monooxygenase; tyrosinase; phenolase; monophenol oxidase; cresolase; catechol oxidase; polyphenolase;
pyrocatechol oxidase;
dopa oxidase
; chlorogenic oxidase; catecholase; polyphenol oxidase; monophenolase; o-diphenol oxidase; chlorogenic acid oxidase; diphenol oxidase; o-diphenolase; tyrosine-dopa oxidase; o-diphenol:oxygen
oxidoreductase; polyaromatic oxidase; monophenol monooxidase; o-diphenol oxidoreductase; monophenol dihydroxyphenylalanine:oxygen oxidoreductase; N-acetyl-6-hydroxytryptophan oxidase; monophenol,
dihydroxy-L-phenylalanine oxygen oxidoreductase; o-diphenol:O2 oxidoreductase; phenol oxidase
However – do not be put off by this apparent complexity. Using nothing more than a banana and a small amount of L-dopa, (which is cheap and non-hazardous), it is possible to demonstrate, in a rapid and colourful method, all the basics of enzymology such as
-
catalytic activity,
-
denaturation by boiling,
-
optimum temperature,
-
effect of pH.
- Using the colourimeter detailed investigations into enzyme kinetics and the effects of inhibitors are relatively simple.
|
There are no hazards associated with the basic investigations described here.
Good laboratory practices should be observed.
|
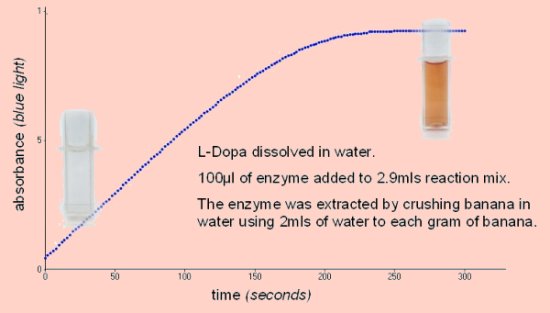
Absorbance vs Time
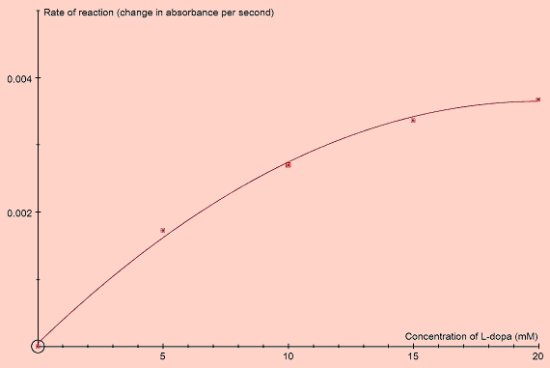
Effect of changing substrate concentration
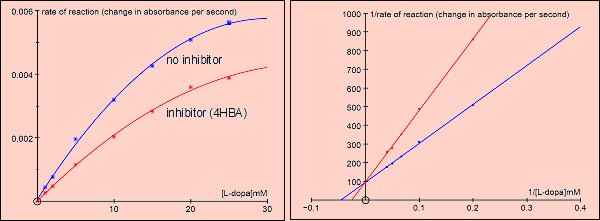
Competitive inhibition by PHBA (4-hydroxy benzoic acid)
Preparing an extract of the enzyme
A variety of animal and plant sources have been used as sources for the enzyme. We have used banana and potato but other sources could be tried and there is potential for extended investigation.
The simplest source is banana. It can be crushed or blended using 2 cm3 of water to each gram of fruit. Filtering through muslin will remove large particles and the extract can be centrifuged to remove remaining debris. However neither of these is essential and a perfectly good preparation can be obtained simply by crushing the banana in water with a fork.
A higher yield of enzyme can be obtained from potato skin. The method is essentially the same as for banana and is easiest if a blender is used, though the skin can be ground in a pestle.
A buffer can be used instead of water, (e.g. 0.1M phosphate buffer pH7).
For best results keep everything cold during the extraction and store the extract refrigerated in which case it will keep quite well for a few days.
The reaction mixture
L-dopa can be dissolved in water; the maximum solubility at 25°C is 0.5g in 100cm3, (25mM). [L-dopa M.Wt =197.2]
0.1cm3 of enzyme extract in 3cm3 of 5mM dopa will give a good reaction at room temperature.
In solution dopa will slowly decompose and darken, particularly at pH above 7. Prepare solutions fresh before use and store them cold.
Solutions of L-dopa will keep well if they are acidified, for example by the addition of a drop of 2M HCl. The acid must be neutralised before use, we do this by adding 0.1cm3 of 1M phosphate buffer pH7 to the cuvette.
 Click to download a pdf file containing much of the information about this
topic including lesson plans.
Click to download a pdf file containing much of the information about this
topic including lesson plans.
|