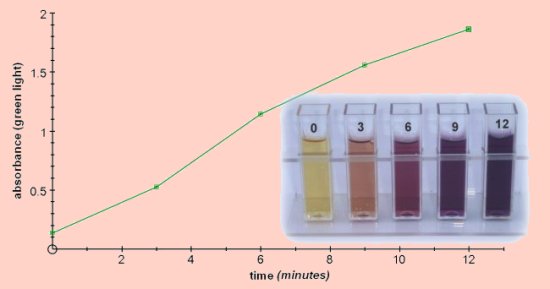
PhosphorylasePotato phosphorylase can be used to demonstrate the synthesis of starch. It can also be used to investigate the equilibrium in a reversible reaction. Phosphorylase, (1,4-α-D-glucan:phosphate α-D-glucosyltransferase, EC 2.4.1.1) is usually associated with the breakdown of starch or glygogen by the release of terminal glucose molecules as glucose-1-phosphate. However, if provided with glucose-1-phosphate and in the absence of inorganic phosphate, the reaction proceeds in the opposite direction and starch is synthesised by the addition of successive glucose molecules to pre-existing polysaccharide chains. 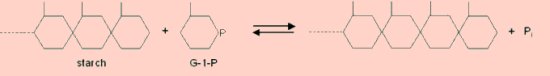 Starch phosphorylase extracted from potato can be used to demonstrate the synthesis reaction in the presence of glucose-1-phosphate. The reaction requires priming with small fragments of partially hydrolysed starch which can grow with the addition of further glucose molecules. It also requires the absence of inorganic phosphate since this will cause the reaction to proceed in the opposite direction. This system can also be used as a good demonstration of reversibility and equilibrium in an enzyme reaction. The direction of the reaction depends on the ratio of glucose-1-phosphate : inorganic phosphate. By adding phosphate during the course of the reaction the direction can be reversed and synthesis of starch becomes degradation of starch. In both of these investigations the reaction can be followed using a colorimeter to measure the starch-iodine complex.
Synthesis of starch
Reversability of the reaction
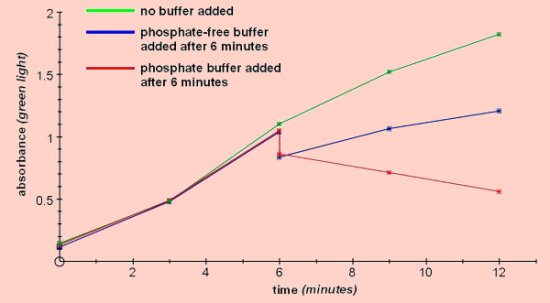
Using a phosphate-free buffer the reaction can be run in the direction of starch synthesis, then by adding a phosphate buffer it can be reversed and the starch begins to be degraded.Comparing this with the effect of the addition of a phosphate-free buffer acts as a control, showing that in this case the reaction will continue to run in the direction of the synthesis of starch.The buffers used were 0.1M citric acid-sodium citrate buffer pH6 and 0.1M phosphate buffer pH6 (see Methods).
Extraction of potato phosphorylase
The resulting liquid can be used as it is or centrifuged to remove suspended particles. Primer
The synthesis of starch by potato phosphorylase can only proceed if the reaction mix is primed by the addition of small fragments of starch at least four glucose molecules in length. A satisfactory primer can easily be prepared by acid hydrolysis of soluble starch. We have found the following method gives good results.
Reaction mixture
0.5cm3 samples were taken and added to 3cm3 of iodine solution containing 0.1M HCl, (iodine stock solution diluted 1:40) The reaction can be carried out at room temperature, the results reported here were all obtained at 25°C.
|