Vitamin CThe determination of the vitamin C content of different drinks, fruits and vegetables is an easy practical and gives good quantitative results. Using known concentrations of ascorbic acid to produce standard curves allows accurate determination of concentration and opportunities for students to practice techniques such as the preparation of dilution series, accuracy and precision. The practical generates results that students find interesting and relevant. Vitamin C, (ascorbic acid), was the first vitamin to be artificially synthesised in 1935, though its history goes back to ancient folk knowledge of the need for fresh plant material and raw animal flesh to prevent disease. Anecdotal evidence from early explorers and particularly from British naval doctors similarly attested to the need for fresh food to prevent scurvy but it was the pioneering experiments of James Lind, a Scottish naval surgeon, in 1753 that gave a scientific basis to these observations. His experiments with different dietary supplements are a very good example for students to illustrate the concept of a controlled experiment. The most important role of vitamin C is in the formation of collagen, the ubiquitous structural protein component found in connective tissues, skin, bones, cartilage, ligaments and elsewhere. Lack of the vitamin eventually leads to the symptoms associated with scurvy resulting from collagen failure. Early symptoms include spongy bleeding gums, liver spots on the skin and lethargy followed in the later stages by loss of teeth, failure of wounds to heal, immobilisation and eventual death. The RDA (Recommended Dietary Allowance) for vitamin C is 40mg/day (UK), 45mg/day (WHO), and 60-95mg/day in USA depending on age and sex. The Tolerable Upper Intake Level in USA is 2000mg/day for a 25 year old male, though independent sources have recommended daily intakes higher than this. The methods for detecting vitamin C generally make use of its anti-oxidant property. A method often used in schools is the reduction of DCPIP from blue to colourless. The method recommended here is the decolourisation of the blue/black starch-iodine complex which we have found as accurate, cheaper and easier than the alternative.
Colourimetry
The amount of vitamin C in different fruits and vegetables can be compared, the effects of boiling on vitamin C, changes during ripening, etc.
Titration
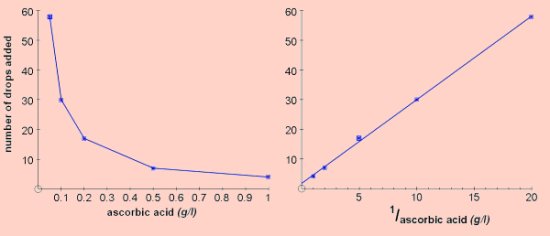
The results shown below were obtained by counting the number of drops needed to decolourise 3cm3 of starch-iodine using a plastic pipette. This method is perfectly adequate and is easy to use with groups of students. The graph on the right is the reciprocal plot, 1/concentration vs number of drops.
Starch-Iodine - The blue starch-iodine complex will decolourise in the presence of vitamin C.
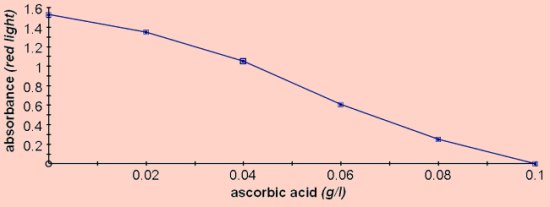
For colorimetry this should be diluted 1:4 with water to give an absorbance between 1 and 1.5 using red light. DCPIP - 2,6-Dichlorophenol-indophenol - DCPIP will decolourise in the presence of vitamin C.
Add 1cm3 of this solution to 99cm3 of water to make a 0.01% solution of DCPIP.
|