Iodine Clock ReactionClock reactions investigate reaction kinetics by mixing substances which, after a delay, suddenly start to change colour. The iodine clock was first described by Hans Heinrich Landolt in 1886. A number of different clock reactions have been described, some of which are variations of the iodine clock. For the clock reaction described here one solution contains hydrogen peroxide and sulphuric acid, the other contains potassium iodide, sodium thiosulphate and soluble starch. When these are mixed triiodide ions are slowly produced. These will react with starch producing the characteristic blue starch-iodide complex. However the presence of thiosulphate rapidly converts the triiodide to iodide so the blue complex does not start to form until all the thiosulphate has been exhausted.
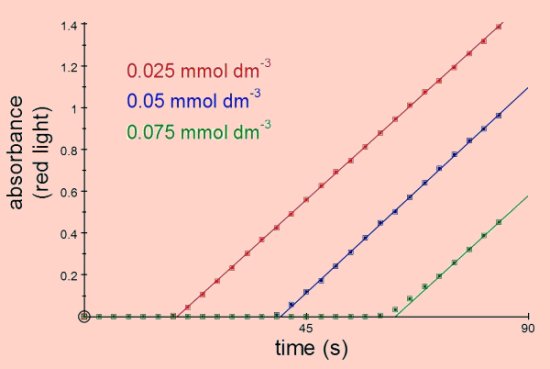
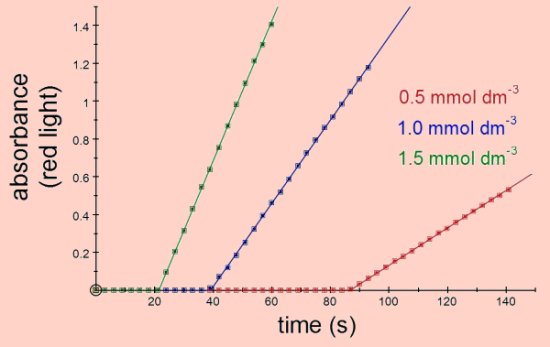
The time taken for the blue colour of the starch-iodide complex to start appearing depends on the rate of the initial reaction and on the concentration of the sodium thiosulphate. With a fixed concentration of thiosulphate the time taken for the blue colour to first appear is usually used as the indicator of the reaction rate, hence the name 'clock reaction'. Using the colorimeter the rate of reaction can be obtained from the slope of the line of absorbance against time, irrespective of the concentration of thiosulphate. In fact the kinetics of this reaction can be found without the need for the addition of sodium thiosulphate since its only purpose is to delay the appearance of the blue product. The rate of the reaction will depend on temperature, concentration of potassium iodide, concentration of hydrogen peroxide and concentration of H+ ions (i.e. concentration of acid).
Absorbance vs Time
The reaction was performed at room temperature using the reaction mixture described in ‘Methods’.
Vary the concentration of thiosulphate
Vary the concentration of potassium iodide
Similar results can be obtained by varying the concentration of hydrogen peroxide, concentration of acid or by varying the temperature.
The following concentrations at room temperature gave a time of about 40 seconds for the 'clock' (i.e. the time from mixing equal volumes of the two solution until the first appearance of the blue colour of the starch-iodide complex).
|
|||||||||||||||||||||