Reaction of potassium iodide with ferric chloridePublished descriptions of this reaction are quite variable and many are certainly incorrect. There is a comprehensive treatment of the reaction here which suggests that the reaction is second order with respect to iodide and first order with respect to ferric ions. This is consistent with the results we obtained - shown below.
There are no hazards associated with the reagents at the concentrations described here. Good laboratory practices should be observed.
Vary the concentration of potassium iodide (KI)
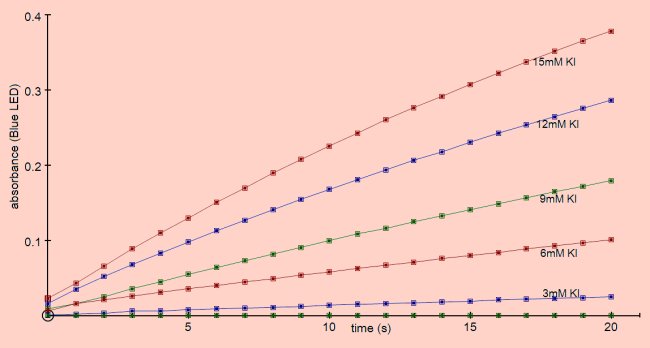
The reactions were performed at room temperature using the reaction mixture described in ‘Methods’.
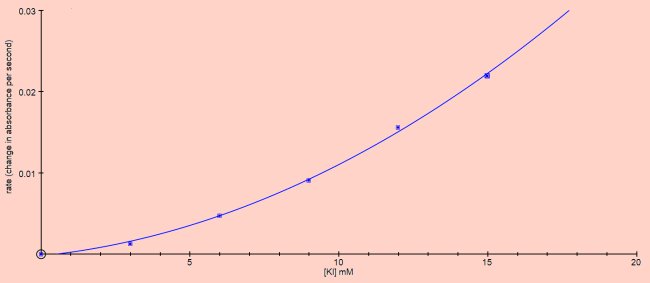
By taking the rate of reaction from the initial slopes of the curves on the graph above the effect of concentration of KI vs rate of reaction can be plotted.
This looks like second order reaction kinetics. Vary the concentration of ferric chloride (Iron(III)chloride, FeCl3)
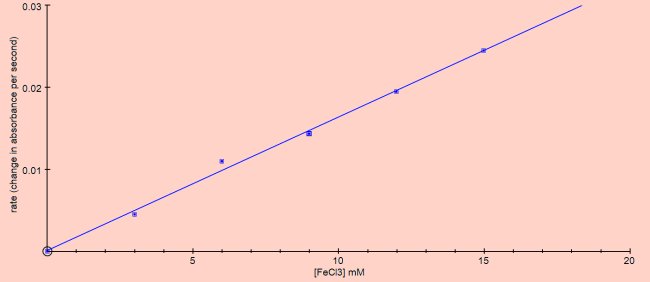
The reactions were performed at room temperature using the reaction mixture described in ‘Methods’.
This looks like a first order reaction so the rate equation would be:
[or perhaps rate = k [KI]1.7[FeCl3]1]
0.03M (30mM) solutions of KI and FeCl3 were prepared. the reaction mixture was 1cm3 of each in a 4cm3 cuvette (appropriate dilutions of whichever reactant was being varied were prepared and added as 1cm3 aliquots). The reaction is quite rapid so it is important to cap, the cuvette, invert it to mix the contents and start taking readings as quickly as possible. Lower concentrations would give slower reactions which might be more easy to manage.
|